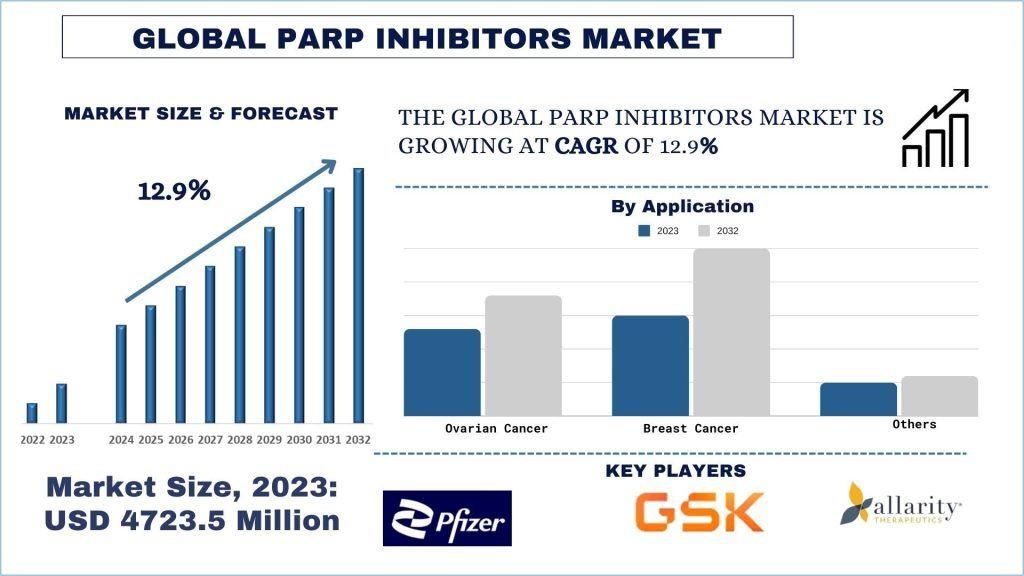
According to a new report by UnivDatos, the PARP Inhibitors Market is projected to reach USD 13,835 million by 2032, expanding at a robust CAGR of 12.9% during the forecast period. PARP (Poly ADP-ribose Polymerase) inhibitors are a class of targeted cancer therapies that block the activity of PARP enzymes responsible for repairing single-strand DNA breaks. By inhibiting these enzymes, PARP inhibitors prevent cancer cells from repairing damaged DNA, ultimately leading to cell death—particularly in tumors with existing DNA repair deficiencies.
PARP inhibitors have demonstrated strong clinical efficacy in the treatment of multiple cancers, especially those associated with BRCA1 and BRCA2 gene mutations, including breast, ovarian, prostate, and pancreatic cancers. These therapies are increasingly being used as monotherapies as well as in combination with chemotherapy, radiation therapy, immunotherapies, and other targeted treatments. While side effects such as nausea, fatigue, and anemia remain a concern, the therapeutic benefits of PARP inhibitors continue to outweigh these challenges, positioning them as a critical advancement in modern oncology.
Access sample report (including graphs, charts, and figures): https://univdatos.com/reports/parp-inhibitors-market?popup=report-enquiry
Growing Clinical Pipeline Driving Market Expansion
The report identifies the expansion of the clinical pipeline as one of the primary drivers fueling growth in the PARP inhibitors market. A rising number of PARP inhibitor candidates are currently undergoing clinical trials across various cancer indications, reflecting their expanding therapeutic potential beyond BRCA-mutated tumors.
Ongoing research efforts are increasingly focused on combination therapies, which aim to enhance treatment efficacy, overcome drug resistance, and improve patient survival outcomes. The growing pipeline underscores the pharmaceutical industry’s strong commitment to oncology innovation and continues to attract substantial investments from both public and private stakeholders. As new candidates advance through clinical phases, the scope of PARP inhibitors is expected to broaden significantly, further accelerating market growth.
Olaparib Segment Gaining Strong Market Traction
Olaparib, marketed as Lynparza, remains one of the most prominent and widely adopted PARP inhibitors in the global market. Developed by AstraZeneca in collaboration with Merck & Co., Olaparib was the first PARP inhibitor to receive FDA approval in 2014 for the treatment of advanced ovarian cancer. Since then, its approved indications have expanded to include breast, pancreatic, and prostate cancers, reinforcing its position as a benchmark therapy in this drug class.
Olaparib’s clinical success has paved the way for further innovation in PARP inhibition and encouraged other pharmaceutical companies to intensify R&D efforts in this space. Notably, combination strategies involving Olaparib and other targeted or immunotherapies are gaining increasing attention for their potential to improve efficacy and address resistance challenges. For example, in April 2024, the U.S. FDA approved a new indication for Olaparib in combination therapy for the treatment of advanced prostate cancer, further strengthening its market presence.
Click here to view the Report Description & TOC: https://univdatos.com/reports/parp-inhibitors-market
Conclusion
The PARP inhibitors market has emerged as a vital segment within the global oncology landscape, supported by the rising incidence of cancer, advancements in genetic testing, and the growing adoption of personalized medicine. These therapies have demonstrated substantial clinical value, particularly for patients with BRCA mutations and homologous recombination deficiencies, making them an essential treatment option across multiple cancer types.
Leading pharmaceutical companies such as AstraZeneca, GlaxoSmithKline, Pfizer, and Clovis Oncology continue to drive market growth through strong clinical pipelines, strategic collaborations, and ongoing research into next-generation and combination therapies. Favorable regulatory pathways and accelerated approval processes for breakthrough oncology drugs further support market expansion.
Despite challenges related to high treatment costs, potential side effects, and the emergence of drug resistance, continued innovation remains a key priority. Efforts focused on biomarker identification, resistance management, and novel combination strategies will be critical in sustaining long-term growth and improving patient accessibility and outcomes in the PARP inhibitors market.
Contact Us:
UnivDatos
Contact Number - +1 978 733 0253
Email - contact@univdatos.com
Website - www.univdatos.com
Linkedin- https://www.linkedin.com/company/univ-datos-market-insight/mycompany/