"Global Demand Outlook for Executive Summary Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Size and Share
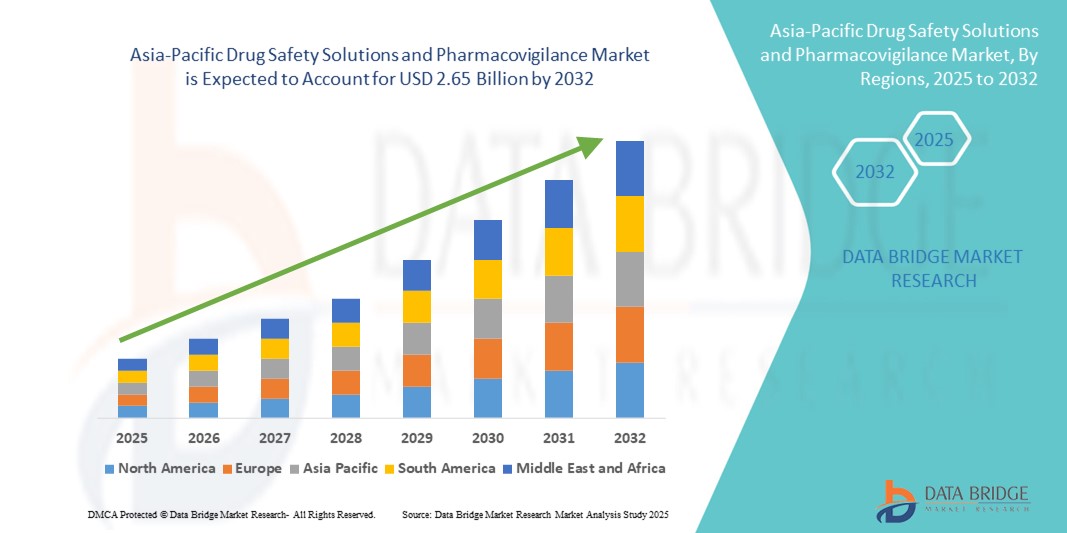
- The Asia-Pacific drug safety solutions and pharmacovigilance market size was valued at USD 1.42 billion in 2024 and is expected to reach USD 2.65 billion by 2032, at a CAGR of 8.10% during the forecast period
Global Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market report is a highly visual business report where the key market research findings have been organized, analyzed, and summarized neatly. This report can be communicated more effectively with a team, stakeholders, and customers. With devotion, commitment, a supreme level of resilience, and integrated approaches, this Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market research report has been prepared. This market document analyzes and evaluates important industry trends, market size, market share estimates, and sales volume with which Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market industry can speculate the strategies to increase return on investment (ROI). In the global Asia-Pacific Drug Safety Solutions and Pharmacovigilance business report, the statistics have been represented in the graphical format for an unambiguous understanding of facts and figures.
A persuasive market analysis report provides CAGR values along with their fluctuations for the specific forecast period. Similarly, the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market report contains top-to-bottom analysis and estimation of various market related factors that are incredibly crucial for better decision-making. The report gives a comprehensive explanation of market definition, market segmentation, competitive analysis, and key developments in the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market industry. This market analysis report is framed with the most excellent and sophisticated tools of collecting, recording, estimating, and analyzing market data. The Global Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market research report comprises data that can be very much indispensable when it is about dominating the market or making a mark in the market as the latest emergent.
Get strategic knowledge, trends, and forecasts with our Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market report. Full report available for download:
https://www.databridgemarketresearch.com/reports/asia-pacific-drug-safety-solutions-and-pharmacovigilance-market
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Exploration
Segments
- By Function: The Asia-Pacific drug safety solutions and pharmacovigilance market can be segmented by function into the following categories: fully integrated software solutions, adverse event reporting software, drug safety audits software, issue tracking software, and others. These different functions cater to the diverse needs of pharmaceutical companies in monitoring, reporting, and managing drug safety and pharmacovigilance activities effectively. Fully integrated software solutions offer comprehensive tools for end-to-end pharmacovigilance operations, while adverse event reporting software focuses specifically on capturing and documenting adverse events related to drugs. Drug safety audits software enables companies to conduct audits for compliance with regulatory standards, and issue tracking software helps in managing and tracking safety issues efficiently.
- By Delivery Mode: The market can also be segmented by delivery mode, including on-premise solutions and cloud-based solutions. On-premise solutions involve the installation and maintenance of software on the company's premises, providing greater control and security over data. In contrast, cloud-based solutions offer flexibility and scalability, allowing companies to access pharmacovigilance software remotely and benefit from automatic updates and maintenance. The choice between on-premise and cloud-based solutions depends on factors such as security requirements, IT infrastructure, and budget considerations of pharmaceutical companies in the Asia-Pacific region.
- By End User: The Asia-Pacific drug safety solutions and pharmacovigilance market can further be segmented by end users, including pharmaceutical companies, contract research organizations (CROs), business process outsourcing (BPO) firms, and other healthcare providers. Pharmaceutical companies are the primary users of drug safety solutions and pharmacovigilance services, as they are responsible for ensuring the safety and efficacy of their products in the market. CROs and BPO firms provide outsourced services in pharmacovigilance to pharmaceutical companies, offering cost-effective solutions for managing drug safety data and regulatory compliance. Healthcare providers also utilize drug safety solutions to monitor and report adverse drug reactions for patient safety and regulatory purposes.
Market Players
- Oracle Corporation: Oracle Corporation is a prominent player in the Asia-Pacific drug safety solutions and pharmacovigilance market, offering a comprehensive suite of software solutions for managing drug safety data, adverse event reporting, and regulatory compliance. The company's pharmacovigilance software enables pharmaceutical companies to streamline their pharmacovigilance operations and ensure compliance with global regulatory standards.
- IQVIA: IQVIA is another key player in the market, providing end-to-end pharmacovigilance services, including case processing, signal detection, risk management, and regulatory reporting. The company's integrated pharmacovigilance solutions help pharmaceutical companies in the Asia-Pacific region to enhance patient safety, optimize regulatory compliance, and mitigate risks associated with their products.
- ICON plc: ICON plc is a leading provider of pharmacovigilance and drug safety solutions, offering a range of services such as safety surveillance, medical monitoring, benefit-risk assessment, and aggregate reporting. The company's expertise in pharmacovigilance enables pharmaceutical companies in the Asia-Pacific region to proactively identify and manage safety issues associated with their products, ensuring patient safety and regulatory compliance.
- Parexel International Corporation: Parexel International Corporation specializes in pharmacovigilance and safety solutions, providing support to pharmaceutical companies in risk management, safety reporting, and regulatory submissions. The company's pharmacovigilance services help clients in the Asia-Pacific region to streamline their drug safety processes, comply with regulatory requirements, and maintain a strong safety profile for their products.
The Asia-Pacific drug safety solutions and pharmacovigilance market is experiencing significant growth due to the rising demand for effective monitoring and management of drug safety activities within the pharmaceutical industry. One key insight into the market is the increasing adoption of artificial intelligence (AI) and machine learning technologies in drug safety solutions and pharmacovigilance services. These advanced technologies are revolutionizing the way adverse events are detected, analyzed, and reported, leading to more efficient and accurate pharmacovigilance processes. Pharmaceutical companies in the Asia-Pacific region are leveraging AI-powered solutions to enhance signal detection, automate case processing, and improve risk management strategies in drug safety operations.
Another emerging trend in the market is the emphasis on real-world evidence (RWE) data in pharmacovigilance activities. RWE data provides valuable insights into the safety profile and effectiveness of drugs in real-world settings, complementing traditional clinical trial data. Pharmaceutical companies in the Asia-Pacific region are increasingly incorporating RWE data into their pharmacovigilance strategies to better understand the safety and efficacy of their products post-commercialization. By leveraging RWE data analytics and predictive modeling, companies can proactively identify safety signals, optimize risk management strategies, and enhance patient safety outcomes.
Furthermore, the Asia-Pacific drug safety solutions and pharmacovigilance market are witnessing growing collaborations and partnerships between pharmaceutical companies, technology providers, and regulatory agencies to drive innovation and compliance in drug safety practices. Collaborative efforts aim to standardize pharmacovigilance processes, harmonize data collection and reporting standards, and promote transparency and accountability in adverse event monitoring. By fostering strategic partnerships and alliances, stakeholders in the market can collectively address emerging challenges in drug safety, such as data quality issues, regulatory complexities, and evolving safety requirements.
Moreover, the increasing focus on post-market surveillance and pharmacovigilance in emerging markets across the Asia-Pacific region presents new opportunities for market players to expand their footprint and offer tailored solutions to meet the unique regulatory and operational needs of these regions. With growing regulatory scrutiny and the globalization of pharmacovigilance standards, companies are investing in localized strategies and capabilities to navigate complex regulatory landscapes, cultural differences, and language barriers effectively. By customizing drug safety solutions and pharmacovigilance services to address regional nuances and compliance requirements, market players can enhance market penetration, build trust among stakeholders, and drive sustainable growth in these emerging markets.
In conclusion, the Asia-Pacific drug safety solutions and pharmacovigilance market is undergoing rapid transformation driven by technological advancements, data-driven insights, collaborative initiatives, and regional expansion strategies. Market players need to stay abreast of these evolving trends and leverage innovative solutions to navigate the dynamic landscape of drug safety and pharmacovigilance in the region effectively. By embracing AI, RWE data, collaborative partnerships, and tailored approaches to emerging markets, companies can position themselves as key players in driving patient safety, regulatory compliance, and operational excellence in the Asia-Pacific drug safety solutions and pharmacovigilance market.The Asia-Pacific drug safety solutions and pharmacovigilance market is a dynamic and evolving landscape characterized by various segmentation factors, key market players, and emerging trends shaping the industry. The segmentation of the market by function highlights the diverse range of software solutions catering to different needs within pharmaceutical companies, such as adverse event reporting, safety audits, and issue tracking. This segmentation emphasizes the importance of specialized tools in effectively monitoring and managing drug safety activities. By segmenting the market based on delivery mode, the distinction between on-premise and cloud-based solutions underscores the varying preferences and requirements of companies in terms of security, flexibility, and scalability. The end-user segmentation further delineates the primary users of drug safety solutions, including pharmaceutical companies, CROs, BPO firms, and healthcare providers, reflecting the broad spectrum of stakeholders involved in ensuring drug safety and regulatory compliance.
Key market players like Oracle Corporation, IQVIA, ICON plc, and Parexel International Corporation play pivotal roles in offering comprehensive pharmacovigilance services and solutions to pharmaceutical companies in the Asia-Pacific region. These companies provide a wide array of services ranging from case processing and signal detection to safety surveillance and regulatory reporting, helping clients enhance patient safety, regulatory compliance, and risk management practices. The competitive landscape in the market is characterized by the presence of established players with strong expertise in pharmacovigilance and a track record of delivering innovative solutions to address the evolving needs of the industry.
Emerging trends such as the adoption of AI and machine learning technologies, the utilization of real-world evidence data in pharmacovigilance activities, and the emphasis on collaborations and partnerships for driving innovation underscore the transformative nature of the Asia-Pacific drug safety solutions and pharmacovigilance market. These trends reflect a shift towards more data-driven, efficient, and collaborative approaches in ensuring drug safety and regulatory compliance. Moreover, the focus on post-market surveillance in emerging markets presents new growth opportunities for market players to establish a stronger presence, navigate regulatory complexities, and customize solutions to meet regional requirements effectively.
In conclusion, the Asia-Pacific drug safety solutions and pharmacovigilance market are poised for continued growth and innovation driven by technological advancements, data-driven insights, and collaborative strategies. Market players need to adapt to these changing dynamics by leveraging emerging trends, solidifying partnerships, and tailoring solutions to meet the evolving needs of pharmaceutical companies in the region effectively. By staying at the forefront of market developments and offering value-added services, companies can position themselves as key contributors to enhancing patient safety, regulatory compliance, and operational efficiency in the Asia-Pacific drug safety solutions and pharmacovigilance market.
See how much of the market the company dominates
https://www.databridgemarketresearch.com/reports/asia-pacific-drug-safety-solutions-and-pharmacovigilance-market/companies
Essential Analyst Questions for Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Forecasting
- How much is the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market worth globally?
- What is the expected CAGR for this Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market industry?
- What are the segmentation strategies used in the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market report?
- Which firms are dominating at the global Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market level?
- What nation-level forecasts are available for Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market?
- What multinational corporations are Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market leading in sales?
Browse More Reports:
Global Change Control Management Software Market
Global Charge-Coupled Device (CCD) Imagers Market
Global Cheese Processing Equipment Market
Global Chemical Soil Testing Market
Global Chilli Flakes Market
Global Chlorinated Intermediates Market
Global Chocolate Liquid Extract Market
Global Chondroplasty Market
Global Chronic Cough Market
Global Citrus Oil Market
Global Clinical Alarm Management Market
Global Cloud Computing Insuretech Market
Global Cloud Services Brokerage Market
Global Coatings Raw Materials Market
Global Coconut Milk Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"


