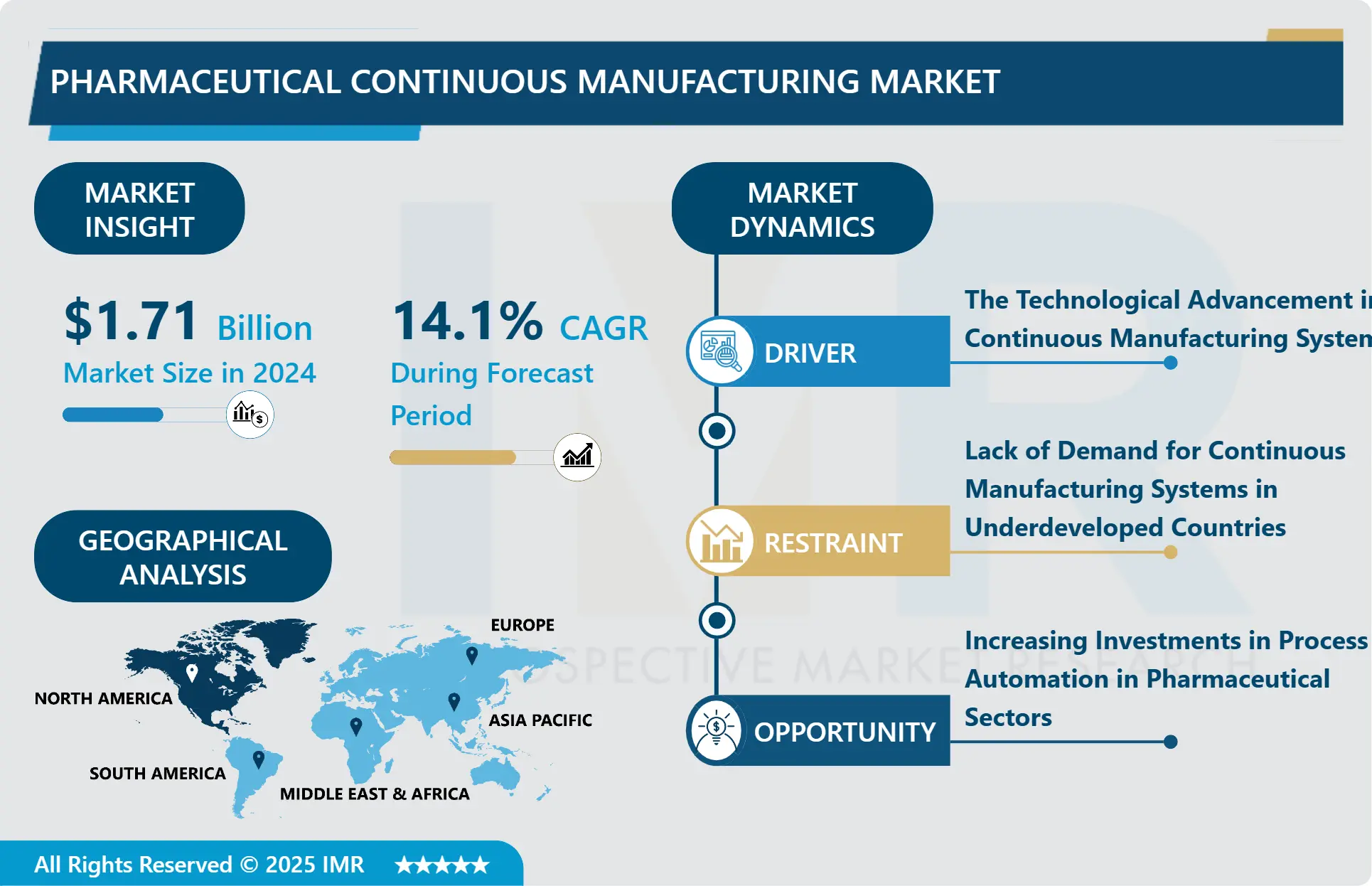
The Global Pharmaceutical Continuous Manufacturing Market Size Was Valued at USD 1.71 Billion in 2024 and is Projected to Reach USD 4.91 Billion by 2032, Growing at a CAGR of 14.1 % from 2025 to 2032.
Pharmaceutical continuous manufacturing (CM), also known as continuous flow chemistry, represents a paradigm shift in drug production, moving away from traditional batch processing toward a fully integrated, continuous flow production line. This advanced manufacturing approach is rapidly gaining traction as it significantly enhances the consistency and quality of pharmaceuticals, reduces production costs, and accelerates the time-to-market for innovative medicines and vaccines. CM offers superior efficiency and safety through end-to-end automation, reducing the risk of human error and enabling real-time monitoring of production processes to ensure compliance with stringent regulatory standards.
The technology is essential for creating active pharmaceutical ingredients (API) and final drug products, requiring less manual intervention and a smaller operational footprint compared to conventional methods. The clear advantages including improved yields, reduced fluctuations in production, and lower operating expenses are driving widespread adoption across both multinational pharmaceutical corporations and Contract Manufacturing Organizations (CMOs). Furthermore, the shift to CM is supported by global regulatory bodies, including the U.S. FDA, which promote its use to improve supply chain resilience and drug quality.
🔍 𝐈𝐧-𝐃𝐞𝐩𝐭𝐡 𝐑𝐞𝐩𝐨𝐫𝐭:
https://introspectivemarketresearch.com/reports/pharmaceutical-continuous-manufacturing-market/
Market Segmentation
The Pharmaceutical Continuous Manufacturing Market is segmented into Product Type, Formulation, Application, Distribution Channel, and End Users. By Product Type, the market is categorized into (Integrated Systems, Semi-Continuous Systems, Controls). By Formulation, the market is categorized into (Solid Formulation, Liquid & Semi-solid Formulation). By Application, the market is categorized into (API manufacturing, Final Drug Product Manufacturing). By Distribution Channel, the market is categorized into (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy). By End Users, the market is categorized into (Research & Development Departments, Pharmaceutical Companies, CMO).
Growth Driver
The primary growth driver for the Pharmaceutical Continuous Manufacturing Market is the technological advancement in continuous manufacturing systems, including the extensive integration of AI and rapid automation in drug methodology. Continuous manufacturing allows pharmaceutical companies to link unit operations, providing greater efficiency, enhanced productivity, and the capability for continuous automated process monitoring. This technological evolution enables manufacturers to produce complex biologics and traditional medicines at a lower cost, addressing the increasing global demand for effective drug production systems and driving investment in modern, high-tech facilities globally.
Market Opportunity
A significant market opportunity is presented by the increasing investments in process automation across pharmaceutical sectors. As demand for new medicines rises, manufacturers are aggressively adopting automated systems to maximize efficiency and reduce time-to-market. Continuous manufacturing systems inherently reduce production variability, improve yields, and lower operational and equipment costs, making automation a key focus area. Major companies are expanding capacity through strategic investments in automated platforms, creating lucrative avenues for technology providers specializing in integrated continuous flow equipment and high-precision monitoring tools.
Detailed Segmentation Title: Pharmaceutical Continuous Manufacturing Market, Segmentation Line below: The Pharmaceutical Continuous Manufacturing Market is segmented on the basis of Formulation, Application, and End Users.
Formulation
The Formulation segment is further classified into Solid Formulation and Liquid & Semi-solid Formulation. Among these, the Solid Formulation sub-segment accounted for the highest market share in 2024. This dominance is primarily due to the segment's early success and regulatory backing, with several fixed-dose drugs already receiving FDA approval for continuous production methods. Key players like Pfizer are developing new products, such as the Portable Continuous Miniaturized and Modular System (PCMM), which are optimized for solid dosage manufacturing. Given that many oral solid dosage products are now off-patent, continuous manufacturing is becoming essential for efficient, smaller-quantity production.
Application
The Application segment is further classified into API manufacturing and Final Drug Product Manufacturing. Among these, the API manufacturing sub-segment is estimated to account for the highest market share in 2024. The continuous synthesis of Active Pharmaceutical Ingredients (APIs) is crucial as it allows for real-time quality control and minimizes batch variations, ensuring a consistently high-quality final product. The shift to continuous API manufacturing is driven by regulatory encouragement and the push for greater chemical process safety and efficiency, making it the foundational step in implementing an end-to-end continuous flow pipeline for drug production.
Some of The Leading/Active Market Players Are-
• Hosokawa Micron Group (United States)
• Robert Bosch GmbH (Germany)
• Thermo Fisher Scientific Inc (United States)
• GEA Group (Germany)
• Coperion GmbH (Netherlands)
• Baker Perkins (United Kingdom)
• Scott Equipment (United States)
• Eli Lilly (United States)
• Chemtrix (Netherlands)
• Novartis AG (Switzerland)
• Siemens (Germany)
• Glatt GmbH (Germany) and other active players.
Key Industry Developments
In August 2023, Siemens acquired the continuous manufacturing software firm, Xtelligent. This strategic acquisition allows Siemens to enhance its portfolio by offering integrated, data-driven solutions that connect hardware and software in continuous flow facilities, providing customers with seamless control and optimization of their pharmaceutical production processes.
In July 2023, GSK and Pfizer partnered with MIT to develop a continuous manufacturing platform for next-generation vaccines. This collaboration underscores the industry's commitment to continuous manufacturing beyond traditional drugs, aiming to accelerate the development, scale-up, and production speed of innovative vaccines using advanced, integrated flow systems.
Key Findings of the Study
• Dominant Segments: Solid Formulation leads by type, driven by regulatory approval, while API manufacturing dominates the application segment in 2024.
• Leading Regions: North America is projected to dominate the market due to high R&D investments and favorable regulatory frameworks.
• Key Growth Driver: The continuous advancement and integration of automation and artificial intelligence in manufacturing systems.
• Market Trends: Strong strategic focus on integrated digital solutions and continuous manufacturing platforms for complex products like vaccines.
👉 To request a sample report:
https://introspectivemarketresearch.com/request/16256
About Introspective Market Research
Introspective Market Research is a global provider of data-driven market intelligence and strategic advisory services. Our analysts and consultants deliver comprehensive reports, actionable insights and customized consulting to clients across chemicals & materials, healthcare, energy, environment, infrastructure, and advanced manufacturing sectors.
Media Contact:
Introspective Market Research
Email: press@introspectivemarketresearch.com
Website: http://www.introspectivemarketresearch.com
Phone: ++91-91753-37569